Study Purpose
Patient Derived Xenografts (PDXs) are models to study cancer. These models are developed by implanting a fresh piece of tumor specimen from a patient into a special type of mouse, which has a limited immune system. These mice act as ‘hosts’ to allow the tumor to grow. Numerous peer-reviewed and published experimental studies have now shown that PDX models maintain features similar to the original tumor from the patient. PDX models can be used to study tumor growth, to evaluate the response to anti-cancer therapies, and to study the mechanisms resistance to anti-cancer therapies.
The purpose of this study is to develop at least 10 PDX models for EGFR driven cancers to be used for research purposes only. That is, these patient derived PDX models will have no immediate benefit to the patient from whom the tumor specimen was obtained. Rather, these PDX models will be used to inform the study of EGFR driven cancers at large.
We are working with Champions Oncology, a company that specializing in PDX models, to create PDX models from EGFR driven cancers. To learn more about Champions Oncology, visit their website at https://championsoncology.com/.
What is a PDX Mouse Model?
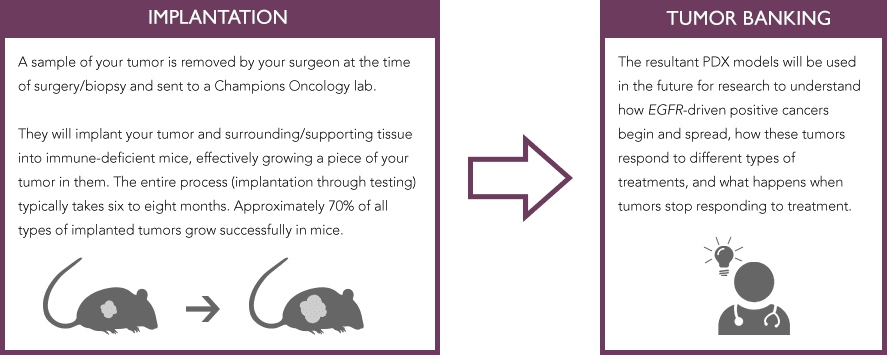
The Champions Oncology team will implant and grow a piece of a patient’s live tumor in immune-deficient mice. With the Champions technology, the PDX tumor can maintain the characteristics of the patient’s tumor, with all of its different cells and connective tissue, inside a living environment (an immune deficient mouse). The resultant PDX models will be used in the future for research to understand how cancer begins and spreads in these tumors, how these tumors respond to treatment and what happens when tumors stop responding to treatment.
The process begins with a piece of your live tumor, so it is best to plan in advance of your biopsy or surgical procedure.
The process is simple:
What Should You Expect in This Study
The research study has certain requirements that must be met. If you do not meet the requirements, you will not be able to participate in this research study. This study will include individuals with an:
- EGFR T790M tumor that has progressed on osimertinib or other EGFR inhibitors like osimertinib (often referred to as 3rd generation or mutant selective inhibitors) or
- EGFR L858R tumor that has progressed on first line osimertinib or
- EGFR exon 19 del tumor that has progressed on first line osimertinib or
- Exon 20 mutation (EGFR Exon 20 insertion or HER2 Exon 20 mutation)
The process begins when you have a surgery or biopsy of your EGFR mutant cancer scheduled. You will speak with a member of our study team to learn about the study activities. After you speak with our team, you will be provided with an informed consent form to review. The consent form explains this research study in detail. You will also provide us with a copy of your pathology and molecular analysis reports to determine your eligibility for this study.
If you are eligible for the study, you will work with the Champions Oncology team to coordinate the collection and shipment of your tumor to their facility. It is very important that you work in collaboration with the Champions team.
- You will call your physician/surgeon and let them know that you want to donate a portion of your tumor to this research study and that Champions Oncology will be in contact with them.
- You will be provided a letter to give to your physician/surgeon notifying them of your participation in this research study.
- You will be in contact with Champions Oncology team as they attempt to train your physician/surgeon on the collection and shipment procedures.