Our Mission
We strive to make lung cancer a survivable disease.
Partnering with the most innovative minds from the world’s premier oncology research institutions, we provide funds, staff, and a team of experts to assist in launching and managing investigator-initiated trials.
As a 501c(3) nonprofit organization, we’re dedicated to forwarding research that can lead to new therapeutics, diagnostics, and a better understanding of lung cancer risk factors and resistance mechanisms.
Explore Our Trials
-
We revolutionized remote, decentralized, observational biomarker trials that push the boundaries of discovery to drive the biggest impact in the most efficient way possible.
See what we're doing. -
Our research has led to a new understanding of oncogene driver mutations and familial risk factors.
Take a look back. -
Lung cancer patients make everything we do possible. Learn more about clinical trials and the power of participation.
Here's how.
The ALCMI Approach to Lung Cancer Science
To maximize our impact and investment, we concentrate our research efforts on 5 areas of focus.
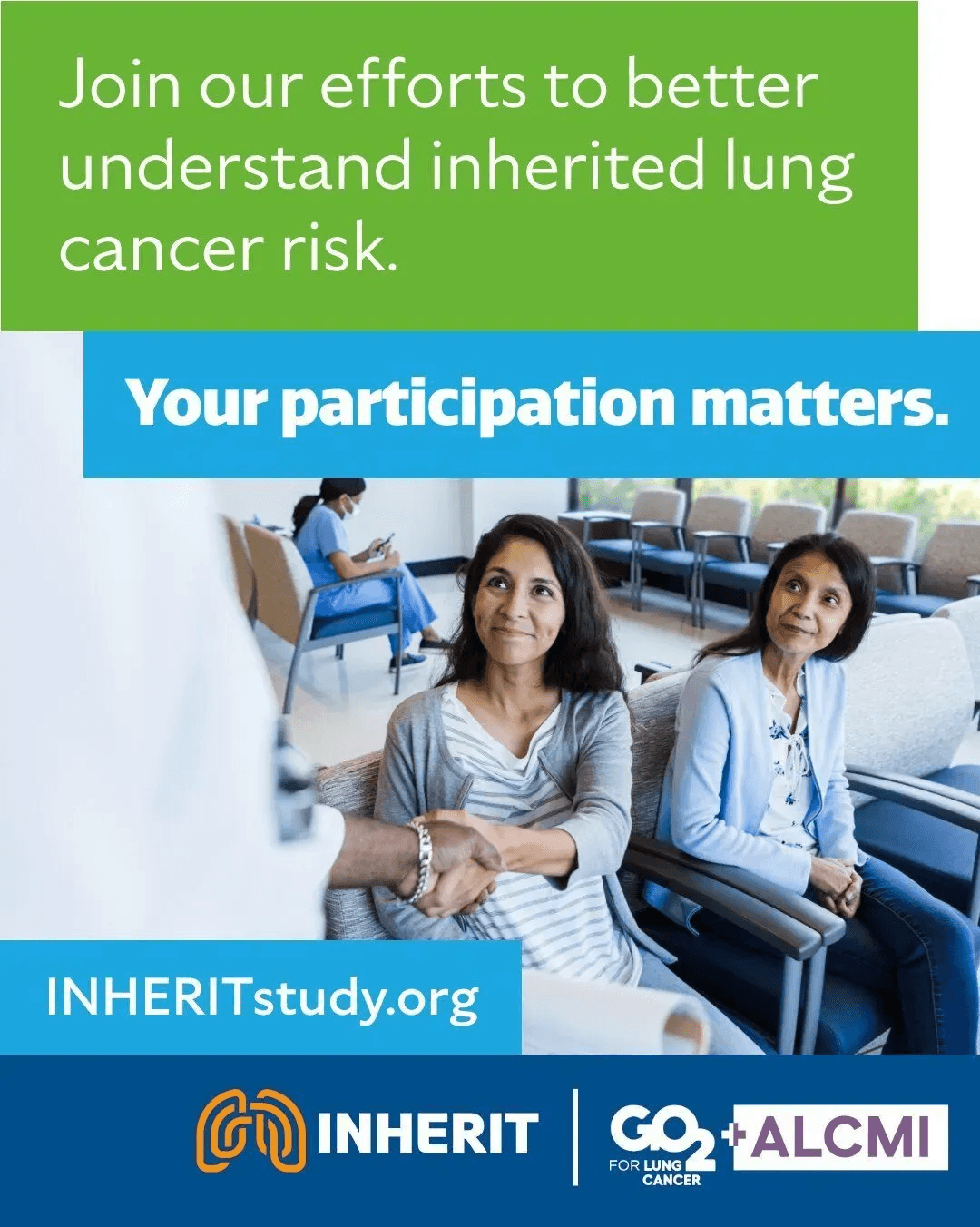
*** Now Enrolling ***
Help us learn more about inherited risk in lung cancer.
Our current study, INHERIT (Investigating Hereditary Risk in Thoracic Cancers), aims to understand better the genetic risk for developing lung cancer. Partnering with Dana-Farber Cancer Institute and GO2 for Lung Cancer, we're investigating why lung cancer sometimes runs in families. We're looking at genetics as well as environmental factors and how these may interact. We hope this information will ultimately improve lung cancer screening, prevention, and treatment.
This is just one of the ways we're working to make lung cancer a survivable disease.

By The Numbers
We invest in lung cancer science.
We're funding critical lung cancer research and providing staffing and guidance to quickly get investigator-initiated trials up and running.
We're proud of our stats, and we're just getting started.
-
Founded
2008
-
Completed Clinical Trials
23
-
Publication Citations and Downloads
13,659
-
Trial Participants
1,754
-
Partners and Sponsors
56
Peer-Reviewed, Published Studies
Our studies have led to numerous publications in the world’s most prestigious peer-reviewed oncology journals.

“My collaboration with ALCMI has been an exceptional experience. Their commitment to advancing lung cancer research is unmatched, and together, we’ve been able to accelerate our understanding of resistance mechanisms to targeted therapies and the development of new therapies for patients in need. ALCMI’s innovative approach and deep expertise have made a significant impact in bridging the gap between research and patient care, offering real hope to those facing lung cancer.”
— DR. BIAGIO RICCIUTI, DANA-FARBER CANCER INSTITUTE
Our Consortium Partners
We're proud to join forces with the world's most prestigious oncology research organizations.